Zeteo’s patient-centric ophthalmic delivery systems are designed for precise, self-administration of preservative free drugs and biopharmaceuticals
AUSTIN, TX – 01 June 2020 — (PR.com) – Zeteo Biomedical LLC announced today the availability of the ZTECH-L™ AquilaMD™ multi-dose ophthalmic drug delivery system. Zeteo’s latest innovative delivery device technology enables patients to precisely self-administer drug and biopharmaceutical therapeutics targeting ocular diseases such as glaucoma, dry eye, macular degeneration, presbyopia, infections and inflammation to the eye. The delivery system is the size of a standard credit card and utilizes a multi-dose cartridge that can be easily reloaded by the patient. The AquilaMD™ can be used in any physical orientation by the patient or caregiver, accurately dispensing a precise, gentle spray to the front of the eye with the touch of a button.
Zeteo’s latest ophthalmic delivery system has an automatic dose counter, requires no priming, does not require a battery to operate and can be configured to dispense a micro-dose to a full standard ophthalmic dose.
ZTECH-L™ AquilaMD™ Multi-Dose Ophthalmic Drug Delivery System

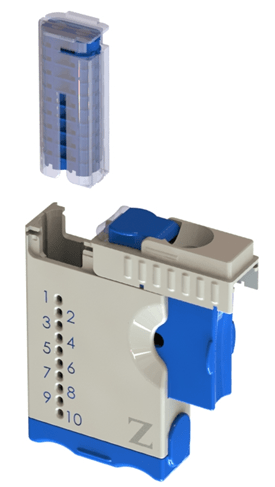
The AquilaMD system utilizes the ZTECH-L™ unit dose Form Fill Seal (FFS) aseptic blister packaging technology to efficiently package each drug dose individually to maintain the sterility of preservative free drug compounds throughout the entire “in use” product cycle. Compared to traditional ophthalmic unit dose packaging, Zeteo’s green FFS packaging technology reduces drug and physical packaging waste flowing into the environment by up to 75%. ZTECH-L™ blister packaging is tamper resistant, provides exceptional barrier properties to protect the drug product during storage and transport and can be stored at room temperature, refrigerated or frozen storage conditions.
The AquilaMD™ delivery system is designed to overcome the challenges of self-administering eyedrops for patients while providing pharmaceutical and biopharmaceutical ophthalmic drug manufacturers with a novel cost competitive drug/device combination product development option for new or existing ophthalmic drug compounds.
